What Is Reverse Osmosis Technology?
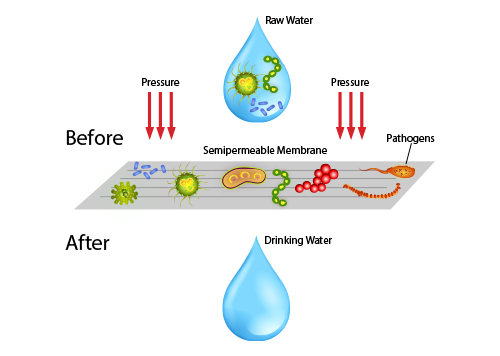
What is reverse osmosis technology reverse osmosis removes contaminants from water down to the molecular level. It works by pushing water through a semipermeable membrane under pressure. This reverses the natural osmosis process, which normally causes contaminants to concentrate on one side of the membrane.
Residential RO systems typically have pretreatment steps to prevent contaminants from clogging the RO membrane. This includes a sediment filter and a pre-carbon block.
What is reverse osmosis and how does it work?
Reverse osmosis is the process that removes salt from seawater. It also makes desalination possible and is used for many other purposes. It is one of humanity’s most important scientific inventions.
The basic working principle of reverse osmosis is that solute molecules move from the area of higher concentration to the area of lower concentration until the two areas have the same concentration. This happens because the solute molecules are trying to find equilibrium with the pure solvent.
To solve this problem, a semipermeable membrane is placed in between the areas of higher and lower concentration. When the water is pushed against the membrane, it pushes on the solute molecules to get them to move toward the pure solvent side. The resulting pressure is called osmotic pressure.
The osmotic pressure is what makes the reverse osmosis process work. This pressure is what drives the water through the membrane and out the other side as clean, pure drinking water. This water can be further purified with a remineralizing filter, which is especially useful for aquarists to create the right amount of salt in their freshwater or saltwater fish tanks.
What is the reverse osmosis technology?
Reverse osmosis is one of the processes that makes desalination (removing salt from seawater) possible. It uses incredibly high water pressure, between 200 and 400 pounds-per-square-inch to push source water through a semipermeable membrane and remove the dissolved salts from it.
A reverse osmosis system starts with a prefilter to remove sediment and chlorine from the water. Then the water is forced through a series of RO membranes to remove dissolved solids and impurities. This can take anywhere from three to five stages depending on the size of the system and its prefilters and postfilters.
The dissolved solids are sent to a drain and the pure water is collected in a storage tank. The RO process will often remove some beneficial minerals from the water, so many systems run it back across mineral beds to add those back in.
Reverse osmosis systems can be used for industrial purposes as well, like making brackish or surface water suitable for industrial boiler feed water, metal finishing, food and beverage, and pharmaceutical processing. This technology is able to reduce total dissolved solids with little to no chemical treatment which is a rare capability for water purification.
Is it healthy to drink reverse osmosis water?
While it is true that reverse osmosis systems remove minerals from drinking water, this doesn’t make the water unhealthy to drink. According to UW Health Family Medicine, the only people who could suffer from mineral deficiency from drinking reverse osmosis water are those who “drink too little water and don’t have a well-rounded diet.”
The fact is that most of the nutrients we need come from food, not water. Drinking a balanced diet and avoiding certain conditions like severe acid reflux or gastrointestinal ulcers can ensure that you get all the minerals your body needs.
Similarly, water filtration methods like sand, gravel, ion exchange, and biofilters can also reduce impurities. However, they’re less effective at removing dissolved contaminants than reverse osmosis. As a result, they may not be as suitable for specific water filtration needs. For example, sand and gravel filters may not be appropriate for water with high mineral content or a hard pH level. Fortunately, RO systems can be combined with other water filtration technologies to address specific health concerns.
What is the working principle of RO?
Reverse osmosis works by applying pressure to one side of a semipermeable membrane. When this pressure is greater than osmotic pressure, solvent particles move from the region with higher solute concentration to the region with lower solute concentration. This is called inverse osmosis.
As the solvent passes through the RO membrane, it encounters contaminants that can bind to the semipermeable membrane and form inorganic deposits. These deposits, also known as membrane fouling or scaling, decrease the life of the membrane and can cause problems like low permeate water flow and high reject stream concentrations.
To prevent this, the water first flows through a prefilter to remove any large particles. Then it moves through a carbon filter to remove chlorine and volatile organic compounds. Finally, it moves through the semipermeable membrane where the majority of the salts and contaminants are removed from the water. This water is then collected in a storage tank where it sits until needed. Some industrial reverse osmosis systems include a fifth stage that sends the water through another postfilter to remove any lingering impurities and to add back in minerals for remineralization.
Why is it called reverse osmosis?
Industrial reverse osmosis systems are often used in desalination plants to generate drinkable sea water, and they can also be found in drinking water systems. This water filtration technique uses a semi-permeable membrane with microscopic pores that allow pure water molecules to pass while keeping bigger contaminants like ionized dissolved salts, organics, bacteria and pyrogens from entering.
The water that passes through the membrane is referred to as permeate, while the concentrated solution left over is known as brine or waste. Compared to other water treatment methods, RO has one of the highest total dissolved solids reduction rates.
To understand the working principle behind reverse osmosis, you can conduct an easy experiment at home by placing a small amount of freshwater and a concentrated solution on opposite sides of a semipermeable membrane. As the pressure applied to the concentration side increases, water molecules will move from the more concentrated solution into the less concentrated one until they reach equilibrium on both sides. Most residential RO systems use three to five stages of filtration before the final purified water is delivered through a dedicated faucet.
Why do we need reverse osmosis?
Reverse Osmosis is a very effective industrial water filter because it removes a wide range of contaminants. It is able to eliminate even the most stubborn of chemicals, such as lead, cadmium, mercury and arsenic. In addition, it can even reduce the amount of salt in the water by up to 80%. Industrial RO systems use between 200 and 400 pounds per square inch (psi) to push the water through. This high level of pressure is necessary to create the force needed for this filtration technology.
Despite being very effective, there are some drawbacks to reverse osmosis. Most importantly, it removes beneficial minerals from the water. This can result in a lower pH and may be corrosive to pipes. To address this, many systems include a remineralizing filter to add these essential nutrients back into the water.
The other major concern is the risk of biological contamination. This is known as biofouling and can occur when microorganisms make their way onto the membrane. The best way to prevent this is through UV disinfection. On a smaller scale, reverse osmosis can be used in homes as a whole-house water filter. This is typically installed in the garage and ties into your home’s water line to provide RO filtered water to all of your household appliances.
Why is reverse osmosis not used?
Reverse Osmosis is an effective water filtration system that removes a wide range of contaminants, including sediment, organic matter, heavy metals and dissolved inorganic compounds. It also removes disinfection byproducts and volatile organic compounds. It is particularly good at reducing the levels of arsenic, nitrate, perchlorate and hexavalent chromium in drinking water.
Revers Osmosis is commonly used in large scale industrial settings for things like ocean desalination and removing toxins from drinking water. It’s cost-effective and relatively environmentally friendly compared to other methods of treatment.
However, on a smaller scale, it’s not so practical. For one thing, the process requires a lot of water. It can also take a long time to produce clean water. Moreover, reverse osmosis systems typically target water impurities indiscriminately, so they can also remove beneficial minerals from the water. Additionally, RO systems usually waste a significant amount of water in the form of drain water. To address this, many RO systems are designed with an air gap faucet to prevent the dirty drain water from returning to clean drinking water.
Is reverse osmosis just diffusion?
Reverse osmosis is a process that uses a semipermeable membrane to separate a more concentrated solution from a less concentrated one. The solvent molecules can pass through the membrane but large molecules, like ions and salts, cannot. If the concentration of solute molecules on one side of the membrane is higher than the concentration on the other, osmotic pressure will cause water to flow through the membrane from the more concentrated solution to the less concentrated one.
The water pushed through the membrane is a more pure form of water, with a lower concentration of dissolved solids than the original feed water. This can be used to de-salinate seawater or make brackish water from freshwater, for instance.
This can be useful for removing harmful toxins from drinking water, cleaning up polluted streams, and even recycling waste chemicals. It’s also used by the food industry to concentrate whey and milk, in pharmaceutical manufacturing, and in some cars. Maple syrup is made using reverse osmosis to remove the water from the sugary concentrate. The reclaimed water can be used to run the pumps that force the water through, re-harvesting energy.